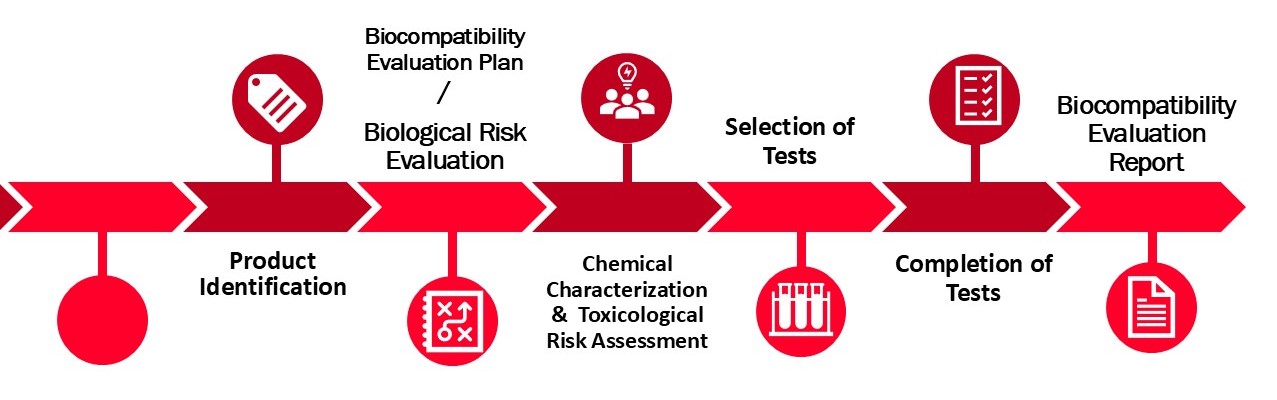
In the complex journey of medical device development, ensuring the safety and compatibility of your device with the human body is paramount. DeSia introduces a comprehensive Biocompatibility Evaluation and Strategy service, designed to navigate the intricate requirements of biocompatibility assessment in line with global regulatory standards, particularly ISO 10993 and ISO 18562 standard series. Our service is meticulously structured to cover all critical aspects of biocompatibility, from initial evaluation to regulatory submission, ensuring your device achieves the highest standards of safety and efficacy.
Comprehensive Biocompatibility Assessment
Our team of experts embarks on a thorough investigation to provide general information about your device, including its intended purpose, users, patient population, indications for use, and any contraindications. This foundational step ensures a clear understanding of your device's application and target demographic, setting the stage for a tailored biocompatibility evaluation.
Device Classification and Material Analysis
We classify your device according to the nature and duration of body contact, following the guidelines of ISO 10993-1. This classification guides the scope and depth of the required biocompatibility assessments. Additionally, our service includes a detailed summary of physical and chemical information, shedding light on your device's manufacturing processes and the materials involved. A meticulous literature search is conducted for each material to identify any potential safety hazards, ensuring a comprehensive assessment of the materials constituting your device.
Biological Data Review and Risk Determination
Our evaluation encompasses a thorough review of existing biological data related to your device, integrating this information to assess potential risks. This holistic approach allows us to identify and address any safety concerns early in the development process, facilitating a smoother regulatory pathway.
Chemical Characterization and Biological Testing Plan
DeSia Clinical Consulting excels in creating detailed chemical characterization and biological testing plans, as dictated by the ISO 10993 series. This plan outlines the specific tests required to demonstrate your device's biocompatibility, ensuring that all regulatory and safety requirements are met comprehensively.
Regulatory Support and Submission
Understanding the complexities of regulatory submissions, we stand by your side in delivering the Biocompatibility Plan /Report to your Notified Body or Competent Authority. Our support extends beyond the preparation of documentation, encompassing strategic advice and assistance throughout the submission process to ensure your device progresses smoothly towards market approval.
Partner with DeSia for Biocompatibility Excellence
Embrace the confidence that comes with DeSia Clinical Consulting's Biocompatibility Evaluation and Strategy service. By partnering with us, you secure a partner dedicated to navigate the regulatory landscape with precision, ensuring your medical device is not only compliant but also poised for clinical success. Let us guide you through the critical steps of biocompatibility assessment, supporting your journey to deliver safe, effective medical devices to the market.