An Overview of the SCHEER’s “Update of the Guidelines on the benefit-risk assessment of the presence of phthalates in certain medical devices covering phthalates which are carcinogenic, mutagenic, toxic to reproduction (CMR) or have endocrine-disrupting (ED) properties”
The Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) has recently released an updated version of its guidelines on the benefit-risk assessment (BRA) of phthalates in certain medical devices. These guidelines specifically address phthalates that are classified as carcinogenic, mutagenic, toxic to reproduction (CMR), or have endocrine-disrupting (ED) properties. The first version of the guidelines was published in 2019, and this is the first revision after five years.
Purpose of the Guidelines
The guidelines serve to provide clarity on the requirements set forth in the MDR, specifically in Annex I, Section 10.4. This section of the MDR deals with substances that might be released from a medical device. According to Section 10.4.1 of the MDR, the use of CMR substances categorized as 1A or 1B, or ED substances, in concentrations exceeding 0.1% by weight (w/w) in a medical device, is only acceptable if a proper justification is provided.
The SCHEER guidelines provide a detailed methodology on how to conduct a BRA to justify the presence of CMR 1A or 1B and/or ED phthalates in medical devices at concentrations above 0.1% w/w. They also outline the process for evaluating possible alternatives to replace these phthalates, including alternative materials, designs, or medical treatments.
What has changed in the first update of the Guidelines?
The guideline's first revision considers the latest regulatory changes and a recent literature review. It is expanded to include chemical profiles of phthalates as phthalic acid esters. A new Methods subsection explains evidence sources and the SCHEER’s Weight of Evidence (WoE) approach.
The evaluation framework has also been updated. The terminology for non-phthalate alternatives shifted from “potential relevant” to “most relevant” candidates. This change will focus on the most suitable alternatives to limit extensive assessments, as well as recommending a minimum of three alternatives for evaluation. Framework now also suggests to characterize medical device compositions based on ISO 10993-18.
The guidelines also introduce classifications for endocrine disruptors, with Category 1 for known or presumed and Category 2 for suspected disruptors, affecting human health and the environment. Lastly, three new sections in the Annexes address exposure to CMR/ED phthalate alternatives, health hazards, and new alternative CMR/ED phthalates for blood bags.
What are phthalates?
Phthalates are commonly used in various industries as plasticizers for polymers, finding applications in products such as coated fabrics, roofing membranes, and notably, medical devices. They are also used in adhesives, paints, inks, and enteric-coated tablets. One of the most widely used phthalates in medical devices is Di-(2-ethylhexyl) phthalate (DEHP), with typical concentrations in plasticized polyvinyl chloride (PVC) ranging from 30% to 40% w/w. Other phthalates, such as dimethyl phthalate (DMP) and diethyl phthalate (DEP), are not used as plasticizers but serve other functions, such as additives in cosmetics, medical devices, and household products.
The reproductive toxicity and endocrine-disrupting effects of several phthalates have been recognized, leading to regulatory measures. These include CLP (Classification, Labelling, and Packaging) regulations, and usage restrictions under regulations like REACH and the MDR.
Benefit-risk assessment and justification of CMR/ED phthalates
When assessing the use of CMR/ED phthalates in medical devices, it is important to consider that alternatives are not limited to substitute substances or materials. Alternatives could include different device designs (such as coating methods, production processes, or using lower concentrations of substances) or even alternative medical treatments or procedures. These alternatives might involve a combination of technical modifications and alternative substances that could replace or eliminate the use of CMR/ED phthalates altogether.
The alternative chosen must provide similar functionality and performance, ensuring no clinically significant difference in the device's performance or the outcome of the alternative medical procedure. This assessment should be grounded in solid scientific justification. To justify the continued use of a CMR 1A or 1B and/or ED phthalate, the manufacturer must clearly demonstrate that the identified alternatives are not suitable for maintaining the device’s functionality, performance, and benefit-risk balance.
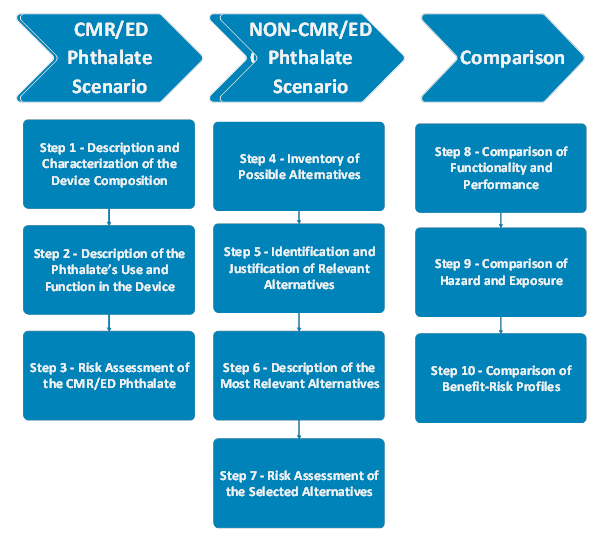
Several aspects should be evaluated when justifying the presence of a CMR/ED phthalate at concentrations greater than 0.1% w/w in a medical device. The assessment consists of 3 main stages. In the CMR/ED Phthalate Scenario, the presence and concentration of CMR/ED phthalates, their function, performance and clinical benefits should be defined. A risk assessment should evaluate the patient exposure, biocompatibility, and hazards of the CMR/ED phthalate. In the Non-CMR/ED Phthalate Scenario, the potential alternatives should be identified, defined, and the risk assessment should be performed, as in the first scenario. Finally, two scenarios should be compared in terms of functionality, performance, hazards and exposure risks. The overall benefit and risk of using the CMR/ED phthalate in the device should be compared with the benefit-risk profiles of the most relevant alternatives.
In summary, the final decision should be based on a comprehensive comparison of functionality, performance, availability, risks, and benefits between the CMR/ED phthalate and its potential alternatives. In order to justify the presence of CMR/ED phthalates, the argument should address why an alternative substance, material, design, or treatment may be unsuitable for maintaining the medical device's functionality and benefit-risk profile. This involves evaluating the importance of any differences in benefits and risks, using value judgments to justify the use of the CMR/ED phthalate over alternatives, and assessing the acceptability of trade-offs. Special consideration must be given to high-risk groups, such as children, pregnant or breastfeeding women, and other vulnerable patient groups.




