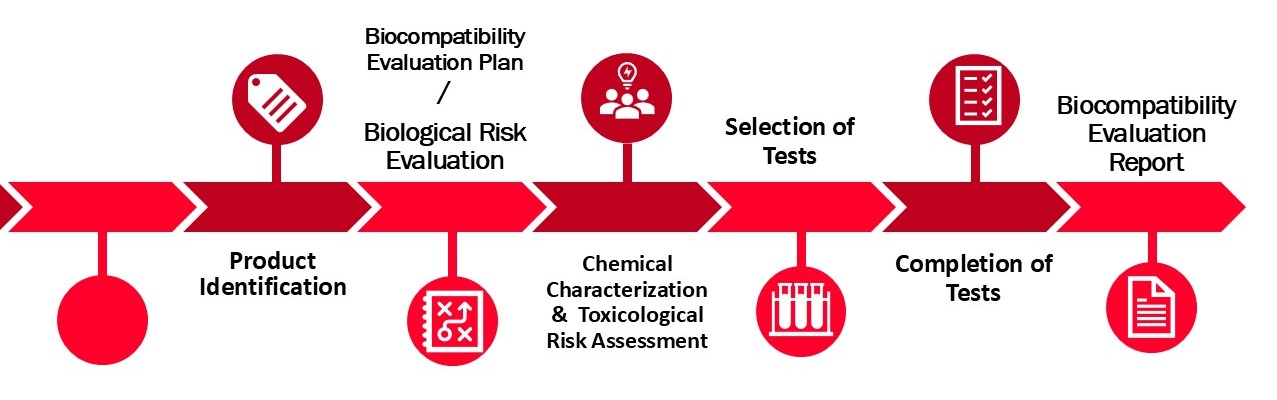
At DeSia, we support you in evaluating the biocompatibility of your medical device in accordance with international standards such as ISO 10993 and ISO 18562.
What We Offer
- Assessment of intended use, user profile, target patient group, and clinical application
- Device classification based on nature and duration of body contact
- Material analysis, including manufacturing process details and literature-based hazard screening
- Review of existing biological data to support risk assessment
- Development of Biological Evaluation Plan (BEP), Toxicological Risk Assessment Report (TRA) and Biological Evaluation Report (BER)
- Support for laboratory selection for chemical characterisation and biocompatibility testing
- Regulatory guidance throughout the submission process
Do you need support with your biocompatibility evaluation? Book your complimentary initial consultation.